The State of Marijuana Legalization in the U.S.
Drivers of the Increasing Incidence of Cannabis Intoxication
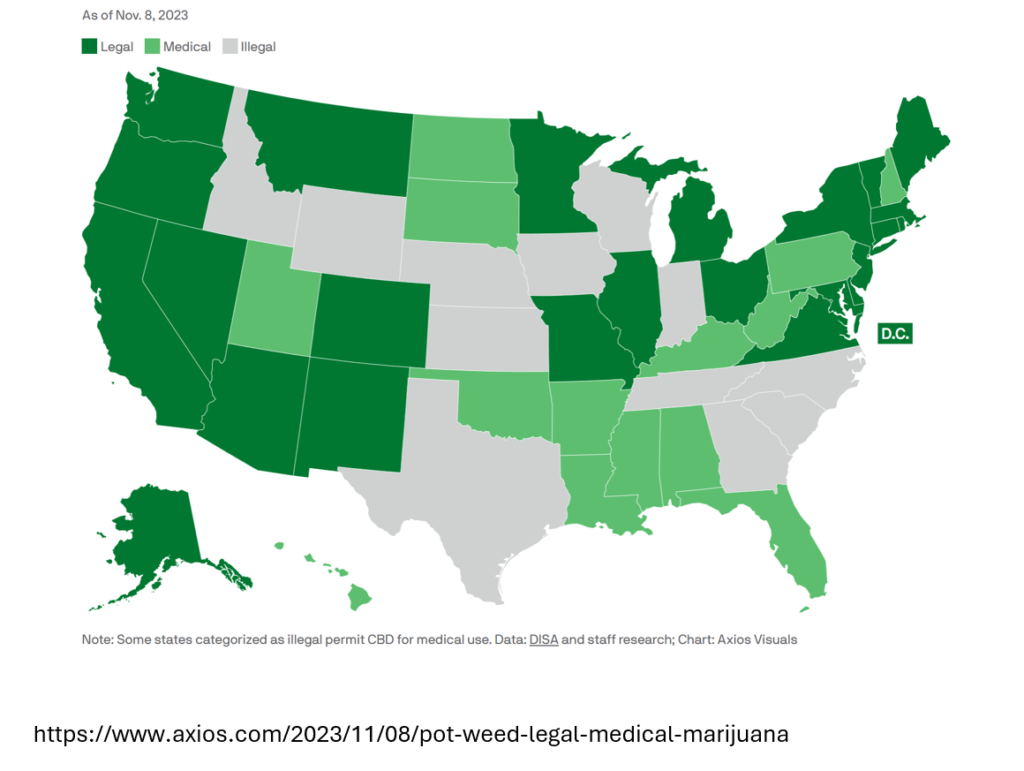
Cannabis is now legal for recreational use in 24 states and legal for medicinal use in 13 states, and more states are likely to legalize cannabis over time. Legalization has not only increased habitual cannabis use but has also served as a driver for increasing emergency room visits due to cannabinoid intoxication, with ingestion of edible and synthetic products being the leading concern.
Children are particularly vulnerable to intoxication given their lower body mass and lack of awareness. Edible cannabis products pose unique risk for pediatric exposure because of their brightly colored packaging and formulation into candies and other sweets. National Poison Data System call volumes show a 40% increase in pediatric related calls in states post-legalization.
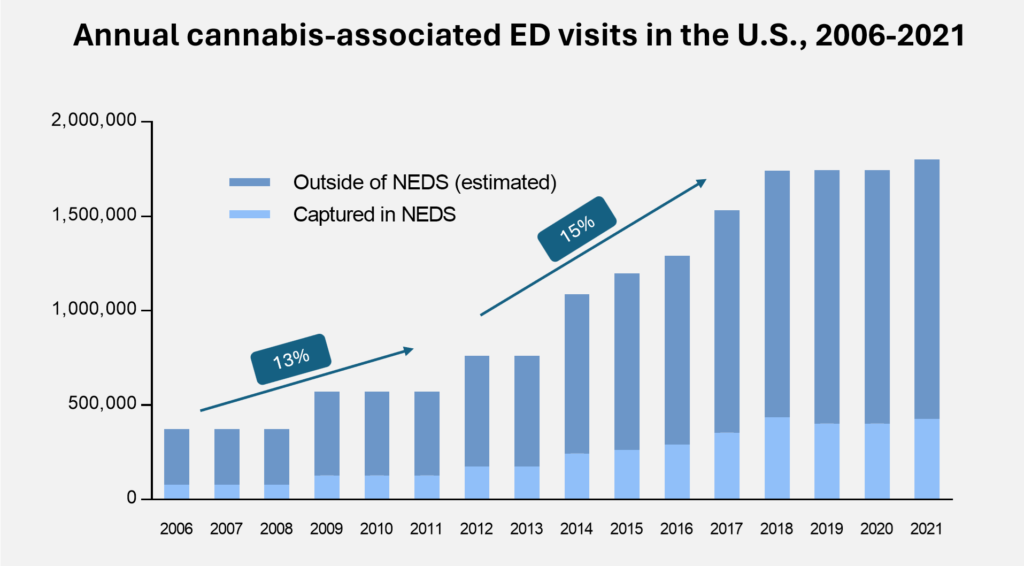
The number of cannabis-associated emergency room visits grew 15% CAGR from 2012 to 2018 as states began to legalize cannabis. In recent years, we witnessed a brief plateau during COVID likely due to the avoidance of hospital exposure, however with the recent support to decriminalize we expect further growth to occur.
A physician survey showed that there is a very strong need for a cannabinoid antagonist to treat cannabis intoxication, with the average score being 7.52 on a scale of 0 (no need) to 10 (significant need), with several physicians noting they “can’t wait for an antidote”.
Pipeline
Given the rise in cannabis-associated emergency room visits and continuing expansion of cannabis legalization, there is an urgent need for a treatment to reverse the symptoms of cannabinoid intoxication.
Our lead investigational therapeutic candidate is ANEB-001, a cannabinoid antagonist that reverses the effects of cannabinoid.
- Products
- Indication
- Preclinical
- Phase 1
- Phase 2
- Phase 3
- Marketed
- ANEB-001
- Cannabinoid Intoxication
- Cannabinoid Intoxication
- Cannabinoid Intoxication
- Cannabinoid Intoxication

